The alcohol module in ORGO
will give you a good review of some of the fundamental
reactions discussed in class and in Chapters 8 and 9. As
you master the chemistry of alcohols, you should try the
Web
of Reactions. 1. How many grams of
K2Cr2O7 in aqueous
H2SO4 are required to oxidize 20
grams of cyclohexanol to cyclohexanone? [This is a
redox reaction from Gen. Chem. Derive the balanced
equation and show your work.] Victor Grignard
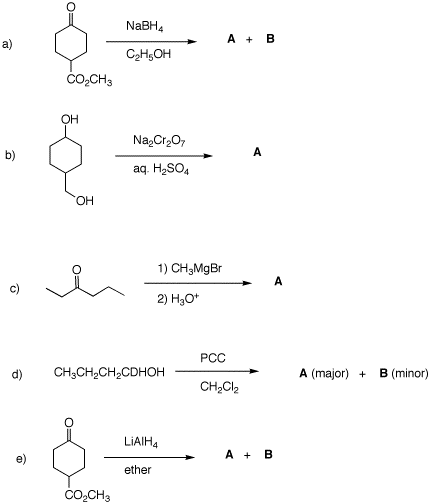
(1871-1935) 2. Predict the products in each of the
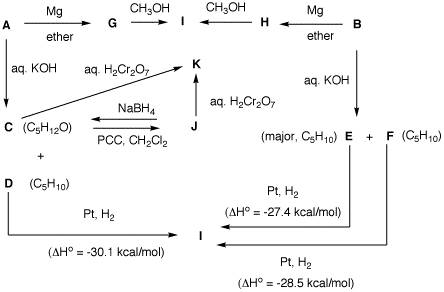
following examples. Justify your answer. 3. Two bottles on a shelf have had
their labels fall on the desktop. Both of the labels read
"C5H11Br". A student decides to run
some reactions on the contents of bottle A and
B to determine the structures of the two
compounds. She also has access, as do you, to
heats
of formation. From the flow
chart below, determine the structure of A and
B and identify C-I. [Note: The mixture
C and D is derived from A.] Show
your reasoning. 4. When organometallic reagents add to
aldehydes and ketones, there is only one addition.
Aldehydes give secondary alcohols and ketones afford
tertiary alcohols. Esters
(RCO2CH3), which are derivatives of
carboxylic acids and are at the same oxidation level as
each other, undergo addition of an organometallic reagent
(R'M) twice to yield a tertiary alcohol,
RR'2COH. The reaction cannot be stopped after
the first addition. It is also true that carboxylic acids
are at a higher oxidation level than aldehydes by two
electrons. Provide an explanation for these results and
provide a mechanism for the process.
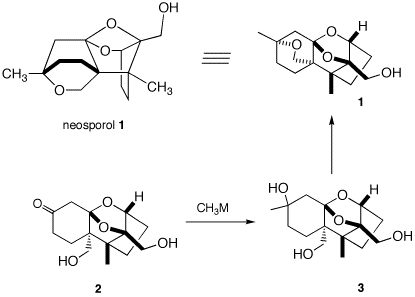
5. Neosporol (1), which is
shown in two views, was successfully synthesized from
racemic ketone 2, whose synthesis is well beyond
the scope of this question. The immediate problem was to
convert ketodiol 2 into triol 3. [The
fact-oid-s have been altered slighted to facilitate the
question. (J. Am. Chem. Soc., 1993,
115, 2581) ] When an excess of methyllithium
was used to convert the ketone function of 2 into
the tertiary alcohol of 3, only ketodiol 2
was isolated upon aqueous workup. a) What is the minimum amount of
methyllithium required in this reaction?
Explain? b) What events occurred prior to
aqueous work up? What was the fate of the ketone
group? When methyl magnesium bromide was
employed, both 2 and a mixture of the
diastereomers of 3 were obtained. Complete
conversion of 2 to 3 (5/1 mixture of
diastereomeric tertiary alcohols) was effected cleanly
with the cerium reagent,
CH3CeCl2. c) Draw the structures of the two
diastereomers of 3. d) Provide conditions and a mechanism
for the conversion of 3 to 1. Is it
necessary to separate the diastereomers of 3 prior
to forming 1? 6. Optically-active compound A
(C10H20O2) reacts with
LiAlH4 in ether to form a single
optically-inactive compound B
(C5H12O). Bromide C is
converted into its Grignard reagent D. Reagent
D reacts with A to form optically-active
E (C9H20O) and
(R)-B. What are the structures A-E?
Explain and illustrate.