|
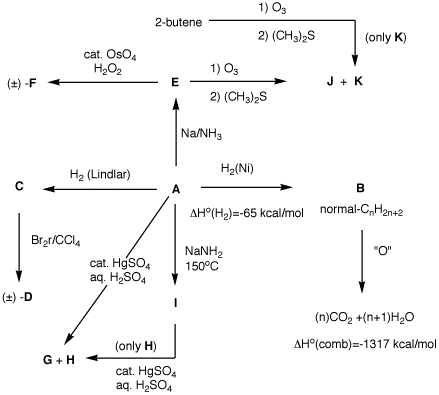
1. Determine the structures A-K. Explain your reasoning.
|
|
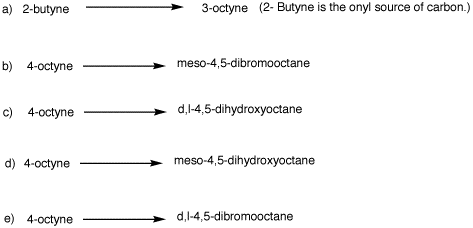
2. Provide reagents for the following reactions. Explain your reasoning.
|
Problem Set 8
Chapter 9, Alkynes
Due: April 7, 2008
Ozone In 1840, Christian Friedrich Schönbein (1799-1868) discovered ozone (Gr.; odorant), the sharp odor produced by electrical discharges. Seven years later (1847) he observed that ozone oxidizes organic compounds but not to their ultimate products of oxidation, carbon dioxide and water. [Two years prior, he had spilled nitric and sulfuric acid on his Frau's apron in her kitchen. The apron, made of cotton, combusted and thus was discovered gun cotton, nitrocellulose. Schönbein also observed that hydrogen peroxide (Threnard; 1818) is oxidized to oxygen gas in the presense of hemoglobin. ] In the period 1903-1916, Carl Dietrich Harries (1866-1923), an assistant to both Hofmann (of the eponymous elimination and rearrangement) and Fischer (of projection and carbohydrate fame) at Berlin, published some 80 papers on the reactions of ozone with organic compounds. His interest was stimulated by the reaction of ozone with rubber, a process that causes rubber to become hard and brittle. These studies led to the analytical and synthetic uses of ozone. From 1904-1916 he was a professor at Kiel. Disenchanted with academic life, he became Director of Research for Siemens and Halske, the German company co-founded by the electrical pioneer, Werner von Siemens, his father-in-law. Not surprisingly, Siemens went into the business of producing ozone generators. The studies of Rudolf Criegee (1902-1975; Karlsruhe) produced a unified mechanism for the process of ozonolysis.
M. Rubin, Bull. Hist. Chem., 2001, 26, 40.
M. Rubin, Helv. Chem. Acta, 2003, 86, 930.
1. Determine the structures A-K.
Explain your reasoning. 2. Provide reagents for the following
reactions. Explain your reasoning.
3. Design a synthesis of muscalure
[(Z)-tricos-9-ene], the sex attractant of the common
housefly, Musca domestica. As a source of carbon you
have available 1-butyne, 1-pentyne and acetylene. You may
use 1-pentyne and acetylene only once, i.e, only seven of
the carbons may be provided by these two alkynes. all
reagents are available. 4. Estimate the heat
of formation of 1-,2-,3- and
4-octyne. Equilibration of any one of these isomers with KOH
at 200oC produces about as much 2-octyne as
3-octyne both of which individually exceed the amount of
1-octyne. However, the amount of 4-octyne is less than the
amount of 2- or 3-octyne. 5. Two bottles are found on a laboratory
shelf labeled "alkyne A" and "alkyne B".
Hydrogenation of A or B over a platinum
catalyst gives the same alkane C. Compound A
reacts with H2 in the presence of Lindlar's
catalyst to form D. Compound D reacts with
O3 to form a single compound E,
C3H6O. On the other hand, compound
B reacts with aq. H2SO4 in the
presence of HgSO4 to give two ketones J
and K. Under the same conditions, A gives
only J. Compound B also reacts with
Na/NH3 to give F, which itself reacts with
Br2/H2O to give a pair of
constitutional isomers, G and H. Treatment of
either G or H with aqueous NaOH gives the same
compound I, C6H12O, that is
also formed by the reaction of F with peracid. What
are the structures of A-K? Explain and illustrate.
[Note: G and H are not distinguished from
one another. Pay attention to
stereochemistry.] 6. The reaction on the right, which was
conducted on three different cycloalkynes, was reported in
1985 by Suzanne Abrams and Angela Shaw of the National
Research Council of Canada. Rather than use NaNH2
as your text suggests, they used the lithium salt of
tetradeutero-1,3-diaminopropane in
tetradeutero-1,3-diaminopropane as a solvent at room
temperature. a) Name the alkynes used in these
experiments. [Note: Not surprisingly, cyclooctyne was
found to be unstable to the reaction
conditions.] b) The base in this experiment is formed
by adding n-butyllithium to the solvent,
tetradeutero-1,3-diaminopropane. Use the pKa
table to explain why this is a sound way to prepare this
base. c) Provide an explanation (mechanism) as
to how each methylene group becomes deuterated. [Hint:
Such reactions are often called "zipper" reactions.
Why?]