Problem Set 5
Chapter 6, Alkyl Halides: Substitution and Elimination
Due: Monday, February 25, 2008
xxxxxxxxxxxxxxxxx
Study #2 and #3 in the Alkyl Halide module and #1 in the Ether module
in ORGO.
Problem Set 5
Chapter 6, Alkyl Halides: Substitution and Elimination
Due: Monday, February 25, 2008
xxxxxxxxxxxxxxxxx
Study #2 and #3 in the Alkyl Halide module and #1 in the Ether module
in ORGO.

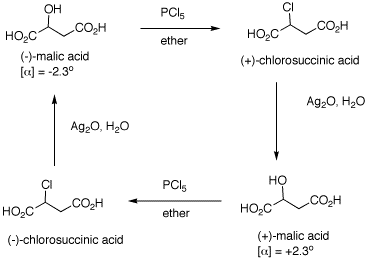
1. The inversion of configuration in an
SN2 reaction is often called a Walden inversion,
named after its discoverer, Paul Walden. In the cycle shown
above, the overall conversion of one enantiomer of malic
acid to the other one must require an inversion of
configuration. Similarly, the same is true of the chloro
acids. More generally, each interconversion of enantiomers
must require an odd number of inversions. The
PCl5 reaction requires a single inversion which
means that the Ag2O reaction involves an even
number of inversions of configuration, namely two in this
instance. (-)-Malic acid is of the
(S)-configuration. a) Show how malic acid, like any alcohol,
might react with PCl5 and then undergo inversion
to form a chloride. Remember that phosphoric acid is a
strong acid and its conjugate base and analogs thereof are
also good leaving groups. b) Silver oxide is an anhydrous form of
AgOH. The carboxylic acid group closest to the hydroxyl
group plays a role in the process. The reaction medium is
mildly alkaline. c) Draw these four enantiomers as Fischer
projections. (-)-Malic acid is of the
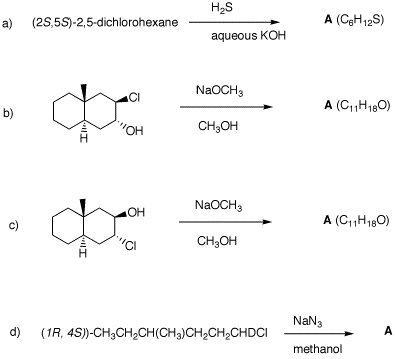
(S)-configuration. 2. In each of the following reactions,
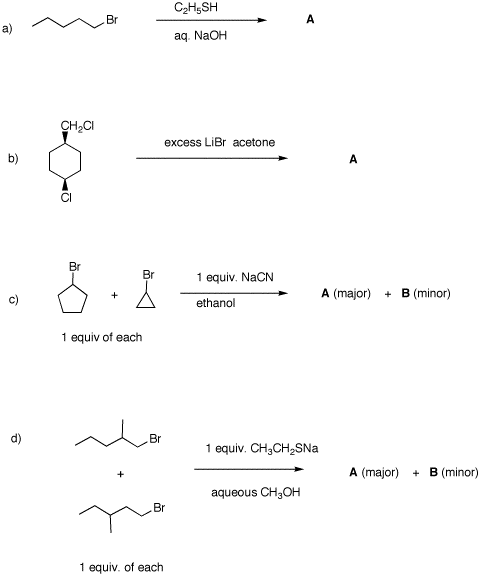
predict the expected products. Explain. 5. Provide the unknown product of each
reaction. In all cases, provide mechanisms and a
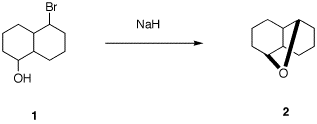
rationale. 6. There are 16 possible stereoisomers of
bromoalcohol 1. Only one of them can produce
optically active cyclic ether 2. a) What is the absolute sterochemistry of
1? b) Provide a mechanism for this reaction
using 3-D illustrations. Explain why your assignment of
stereochemistry is needed. c) Assign RS-descriptors to the
asymmetric carbons in 2. d) What optically active stereoisomer of
1 gives the enantiomer of 2? e) Ether 2 has only 5 chemically
different carbon atoms. Explain.
3. Show how you would convert (R)-2-heptanol into
(R)-2-heptanethiol.
4. (3R,6R)-6-Bromo-3-octanol (A) is expected to
form optically inactive B (C8H16O) upon
exposure to aqueous NaOH. A stereoisomer of A, namely,
C also forms B under the same conditions. Two other
stereoisomers of A, namely D and E as a 50/50
mixture, form optically inactive F, a diastereoisomer of
B. What are the structures of A-F? The structures
D and E are not distinguishable. Explain and illustrate
with mechanisms.