Due: Monday, April
17, 2006
|
The Novice
Level in the NMR
module is an
interactive exercise that will help you learn about
1H and 13C NMR. The estimated order of
complexity is 15, 11, 12, 2, 3, 7, 1, 10, 6, 8, 4, 5, 9,
13, 16, 14, and 17-20. Try to avoid the solutions until
you are truly in need. These exercises are important. I
hope to do some of them in class.
[Note: If
you do not see spectra appear, use Netscape 4.8 available
here.]
|

An α spin
nucleus precessing in a magnetic field
|
|
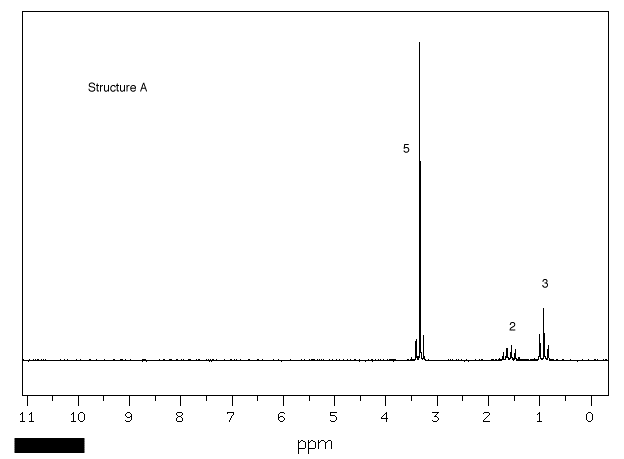
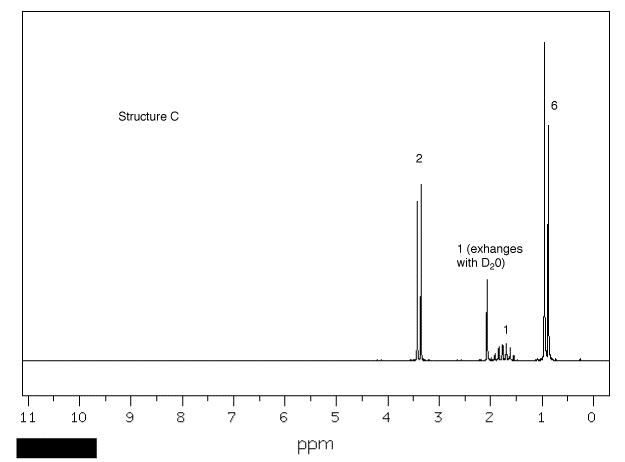
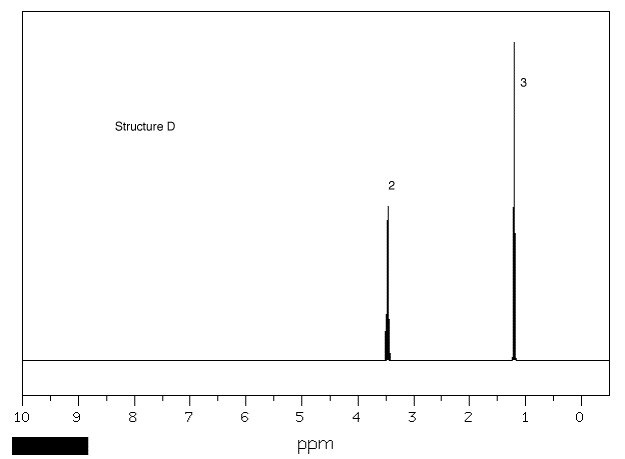
1. Four bottles in a laboratory bear
the labels "C4H10O". A student
records the 1H NMR spectrum of each one
(Spectra A-D). Using the areas number by
resonances, chemical shifts and the multiplicity (as well
as you can discern it), provide the structures of
A-D. Explain. [Click on the spectra for larger
views.] One of the spectra was recorded at 300 MHz,
the others at 90 MHz. Explain which is which.
|
|
2. Predict the number of singlets in
the 13C broadband decoupled NMR spectrum of
each of the following dibromides. Explain.
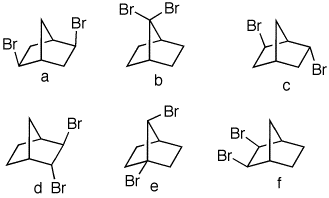
|
|
3. The 1H NMR
spectra of several compounds are given below in standard
line notation. Determine the structure of each one and
the number of resonances that are expected in its
broadband decoupled 13C NMR spectrum. Show
your reasoning. [The first three questions were given
on a recent exam in Chem 425b/525b. Degree
of Unsaturation
is a useful technique in these problems. All compounds
are acyclic. s = singlet; d = doublet; t = triplet; q =
quartet; dd = doublet of doublets; qd = quartet of
doublets.]
A:
C3H3Br; δ 2.33 (1H, J = 2.70
Hz, t) and 3.82 (2H, J = 2.70 Hz, d)
B:
C3H3Br; δ 4.82 (2H, J = 5.85
Hz, d) and 5.85 (1H, t, J = 5.85 Hz)
C:
C3H5NO; δ 3.65 (3H, s) and
4.20 (2H, s)
D:
C3H6O;
δ 1.11 (3H, J =
7.3 Hz, t), 2.46 (2H, J=7.3 and 1.4 Hz, qd) and 9.79 (1H,
J = 1.4 Hz, t)
E:
C4H10O; δ 0.99 (3H, J = 7.5
Hz, t) and 2.37 (2H, J = 7.5 Hz, q)
F:
C4H8O; δ 1.27 (3H, J = 7.0
Hz, t), 3.74 (2H, J = 7.0 Hz, q), 6.46 (1H, J = 14.4 and
6.9 Hz, dd), 4.17 (1H, J = 14.4 and 1.9 Hz, dd) and 3.96
(1H, J = 6.9 and 1.9 Hz, dd)
|
|
4.
This problem needs
to be done on-line and the answers recorded on the
homework. If you do not see the spectra when you click on
the links below, use Netscape 4.8 or go here
to download it. The ancient
tree, Ginkgo
biloba, is claimed to date
back to the age of the dinosaurs (65-150 million years
ago). My neighbors have a female tree in their yard that
drops its rancid fruit during the fall. Butanoic acid
(n-butyric acid) has the smell of rancid butter. It is
one of the odiferous components of the fruit. Several
years ago, I brought some of the fruit into the lab. Dr.
Martha Sarpong (a former orgo TA; Yale Ph.D. 2002)
crushed the fruit, extracted the pulp with hot water,
extracted the cooled aqueous solution with ether,
evaporated the ether and isolated a liquid. The
1H
NMR spectrum and the
13C
NMR spectrum, which were
recorded, are provided. Examine the 1H NMR
spectrum of the extract and you should be convinced that
there is more here than n-butyric
acid (butanoic acid) present.
Note the two triplets at high field of unequal intensity.
a) Calculate their J values in Hz.
b)What are their chemical
shifts?
Expand the region around
d
2.25. It contains two triplets for
-CH2CH2CO2H.
c)What is the J value for each of
these signals and what are the two chemical shifts?
Look at the 13C NMR
spectrum.
d) How many
-CO2H
carbons are there?
The remaining eight signals are
upfield. Three of them belong to n-butyric
acid.
e) The remaining five belong to what
normal chain carboxylic acid? Think about it, then
check
here and here.
f) Assign each signal to one of the
two carboxylic acids. You need not assign the specific
carbon atoms. Expand the spectrum and use the cursor to
get the chemical shift.
|
|
5. In the 1997 Miramax
film, Good
Will Hunting,
[See the commentary by the structure in the previous
link!] Minnie Driver's character Skylar did a
considerable amount of grousing about organic
chemistry in
general and 1H
NMR spectroscopy
in particular. Her complaint about interpreting the
proton spectrum of ibogamine has merit. There isn't a lot
of information in it.
Click here.
a) Look at the
downfield region of the spectrum. What is the large
singlet? What are the chemical shifts of the
sp2 aromatic protons? How many of each are
there?
b) What is the chemical shift, integral, and
multiplicity of the highest field signal in the
spectrum? Assign this signal.
c) What is the sign of the optical rotation of
ibogamine shown here?
On the other hand, if her assignment had been to
interpret the 13C NMR spectrum of ibogamine,
she may not have been so bored. It is much more
informative. Click
here for the
13C NMR spectrum.
d) What is the
strong set of signals around 77 ppm? [It is
actually 3 signals (triplet) and not 4 signals as the
print out at the top of the spectrum
indicates.]
e) Are all of the carbons of ibogamine present in the
spectrum?
f) Draw the structure
of laevorotatory ibogamine.
g) What enantiomer of ibogamine is Will Hunting (Matt
Damon) drawing
here?
h) Assign the singlets
in the region 100-150
ppm to their
respective carbons.That is, assign the taller signals
(mark them with an "x" in part f.) to the appropriate
group of carbons and the shorter signals (mark them
with a "y") to their carbons. You will not be able to
make a specific assignment of individual carbons.
i) Locate the carbons
that absorb at higher field than 40
ppm. Mark them
with a "z". How might they be described?
j) How many carbons remain unassigned? Describe the two
types of carbons that remain. How many of each are
there?
|
![]()