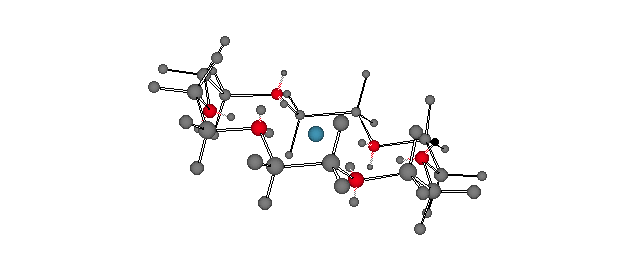
 Potassium cation solvated by the cyclic polyether, 18-crown-6 [18-membered ring; 6 oxygen atoms]. Each of the ethano groups is in a staggered conformation with each of the O-C-C-O dihedral angles at ~60o [gauche]. For a dynamic version, click here. Note that the six oxygen atoms occupy the same spatial arrangement as do the six carbon atoms in chair cyclohexane. The discovery of the crown ethers by Charles Pedersen of DuPont earned him a share in the 1987 Nobel Prize in Chemistry. |
|
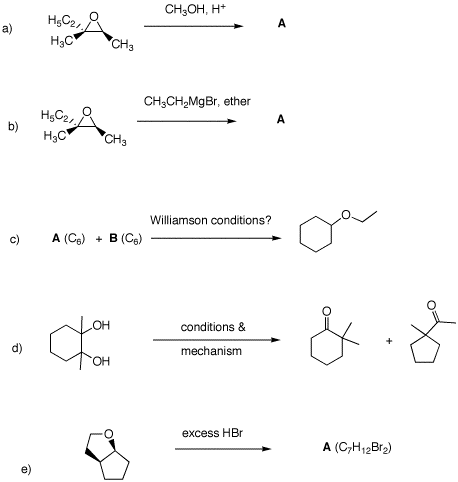
1. In the following problems, provide the missing information. Provide explanations for your choices.
|
|
2. Cartoon A represents the cross-linking a disulfide bond in hair. This property gives hair its natural curl or an artificial "permanent wave". When a solution of ammonium thioglycolate at alkaline pH is applied to the hair, it goes straight to form a dithiol (cartoon B) and disulfide C, which is water soluble and is rinsed away. To restore the curl, the hair is washed with a mild oxidant. Provide a mechanism for the formation of B and C from A, and the formation of A from B.
|
|
3. Consider the structures of tetrodotoxin and monensin on page 337. a) Tetrodotoxin: A guanidinium salt ((NH2)2C=NH2+) has pKa = 13.6. i) Draw tetrodotoxin in the neutral form. [the structure in the text is the zwitterionic stable form.] b) Monesin: The ionophore monensin is biosynthesized by Mother Nature from acetate, propionate and butyrate to form the backbone A of monensin. Each of the three double bonds of A is of the (E)-configuration. They may also be considered trans in relationship to the long chain (i.e; ignore the methyl and ethyl groups in the trisubstituted double bonds). Triene A is stereospecifically epoxided to form triepoxide B, which under the influence of an acid source, is selectively converted into monensin as illustrated by the arrows. i) Compare the structure of monensin with B and determine the stereochemistry of the opening of the epoxides. [One way to do this is to assign R,S-configurations to the six carbons of the epoxides and R,S-configurations of the same carbons in monensin.] |
|
4. Design three independent syntheses of 4-ethyl-4-octanol using three different disconnections. Your sources of carbon contained in the product are limited to ethanol, ethylene oxide and gaseous formaldehyde. All other reagents are available to you. What can you say about the the optical rotation of 4-ethyl-4-octanol in each of your syntheses? |
|
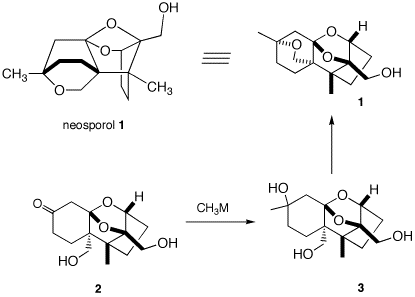
5. Neosporol (1), which is shown in two views, was successfully synthesized from racemic ketone 2, whose synthesis is well beyond the scope of this question. The immediate problem was to convert ketodiol 2 into triol 3. [The fact-oid-s have been altered slighted to facilitate the question. (J. Am. Chem. Soc., 1993, 115, 2581) ] When an excess of methyllithium was used to convert the ketone function of 2 into the tertiary alcohol of 3, only ketodiol 2 was isolated upon aqueous workup. a) What is the minimum amount of methyllithium required in this reaction? Explain? b) What events occurred prior to aqueous work up? What was the fate of the ketone group? When methyl magnesium bromide was employed, both 2 and a mixture of the diastereomers of 3 were obtained. Complete conversion of 2 to 3 (5/1 mixture of diastereomeric tertiary alcohols) was effected cleanly with the cerium reagent, CH3CeCl2. c) Draw the structures of the two diastereomers of 3. d) Provide conditions and a mechanism for the conversion of 3 to 1. Is it necessary to separate the diastereomers of 3 prior to forming 1?
|