|
1. Consider the optically active diastereomer (1S, 2R)-1-bromo-1,2-dideuteriopentane (1). a) What is the major product formed when
1 is exposed to CH3ONa/CH3OH?
Provide the name and mechanism? Is it optically active? |

Biot examining Pasteur's tartrate crystals
|
|
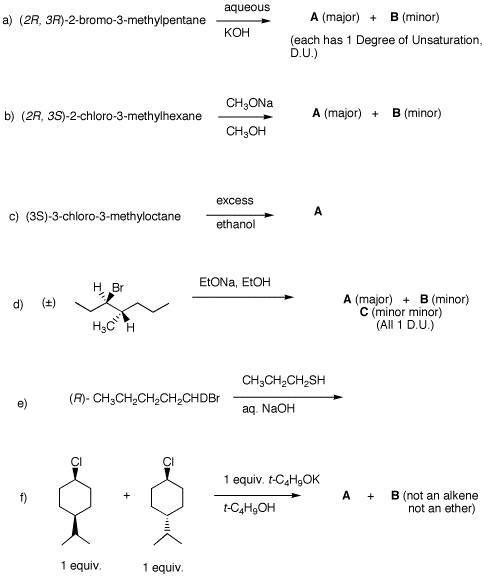
2. Provide the products in each of the following questions and mechanisms for their formation. Pay attention to stereochemistry. Be explicit about enantiomers.
|
|
3. The monochlorination products from the free radical chlorination of 3-methylpentane are isolated. a) Draw all constitutional isomers and diastereomers. Identify the racemates and achiral compounds. Briefly explain why. b) Each chloride reacts with aqueous NaOH. Compounds A and B give the largest ratio of SN2/E2 products (SN2 products C and D, and E2 products G and K, respectively). Compound E gives F (ΔHfo = -14.8 kcal/mol) and G while chloride H gives I (ΔHfo = -15.8 kcal/mol) and G. The ratios F/G and I/G are greater than unity. Compounds F, G and I have one degree of unsaturation. Chloride J reacts much faster with water than do chlorides E and H. Chloride J gives more I than F and more I than K. What are the structures A-K? Explain and illustrate. |
|
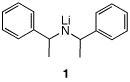
4. A student proposes to study an
elimination reaction. She has access to the hindered base
1, both as its meso diastereomer and as its two
enantiomers. |
 |
|
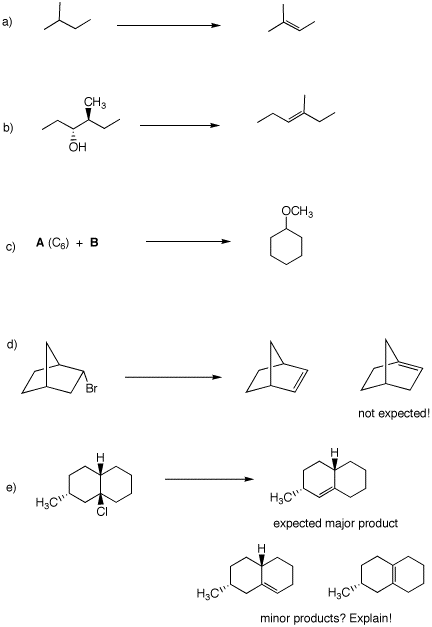
5. Provide reagents for accomplishing the following reactions. some of them may require more than one step. Show any intermediate compounds. Comment briefly on your choices---mechanisms?, etc. Answer queries in d) and e)
|