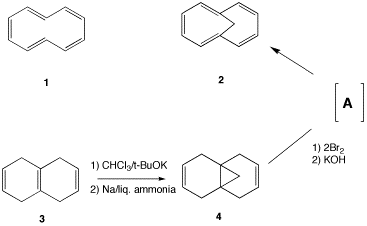
Problem Set 3
Chapter 16
Due: Monday, February 7, 2005
|
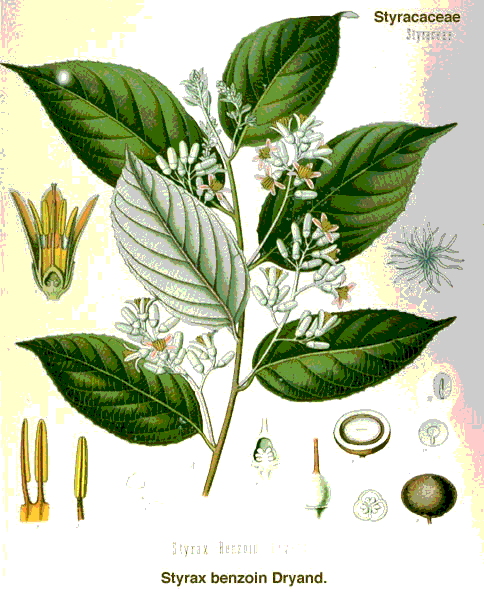
Gum benzoin is a balsamic extract of the Indonesian tropical tree Styrax benzoin. It has been used in both medicine and perfumery. Benzoic acid is a constituent of gum benzoin. Mitscherlich (1834) produced benzene from benzoic acid by heating it with CaO. Independently and earlier, a young Michael Faraday (1925), isolated benzene from coal tar residues and determined its composition, C6H6. Kekule proposed the correct structure of benzene in 1865. His "dream", related at the 25th anniversary celebration of his work, of visualizing the structure of benzene as a snake chasing its tail may well be hyperbole. |
(1829-1896) |