|
The topic of oxidation levels of
organic compounds is addressed in passing on pg. 460. We
will consider the issue in more detail as described in
Oxidation
Levels.The alcohol module in
ORGO
will give you a good review of some of the fundamental
reactions discussed in class and in Chapters 10 and
11. 1. How many grams of CrO3
are required to oxidize 20 grams of ethanol to acetic
acid? Show work. 2. Compound A, C9H18O2, reacts with 2 equivalents of Grignard reagent B to form C (C5H12O) and D (C6H14O). Compound C reacts with aqueous chromic acid to form compound E (C5H10O). Exposure of E to B forms D. Compound D reacts with catalytic H2SO4 to form F, which, upon ozonolysis and dimethyl sulfide reduction affords a single ketone G. Identify the structures A-G. Explain and illustrate. |
Victor Grignard (1871-1935) |
|
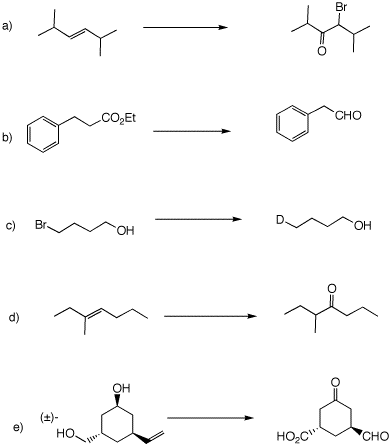
3. Provide reagents necessary to complete each of the following reactions. No one can be accomplished in a single step.
|
|
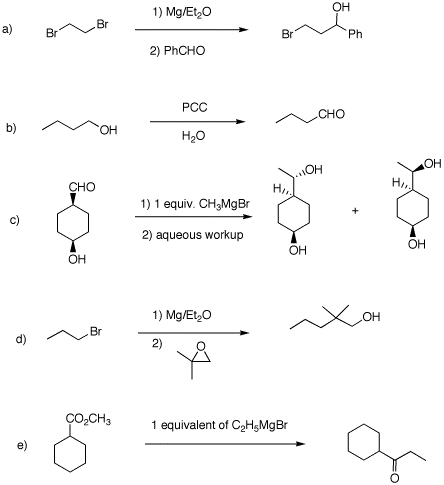
4. Each of the following reactions has a fatal flaw. Comment.
|
|
5. Identify the compounds A-Q in the following "maze" of reactions. Fore a one-page copy of the maze, click here.
|
|
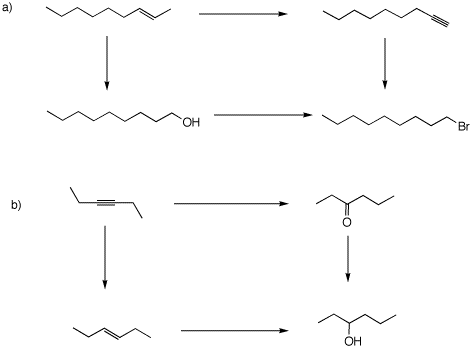
6. In the following two problems, two pathways are provided for converting the starting material into the product. Thus, the total change in electrons (change in oxidation leve or state) by both pathways must be the same. Over each arrow, show the change in oxidation state: 0 e; +n e; -n e. Secondly, provide the reagents necessary to achieve these transformations. The Web of Reactions will be of help.
|