Problem Set 6
Chapter 7
Due: Monday, October 17, 2005
Do problems 2-4 in the Alkyl Halide module in ORGO. They need not appear on your homework.
|
1. Read Degrees (Elements) of
Unsaturation here
and/or here.
How many degrees of unsaturation are present in
C6H8Br2ClN3O2S? |
Paul Sabatier 1912 Co-Nobel Prize in Chemistry Hydrogenation by Metal Catalysis |
|
d) If cycloheptane and
(Z)-cycloheptene were strain free, what would be
there respective heats of formation? What would be the heat
of hydrogenation? |
|
3. Hydrocarbon A (ΔHfo
= -45.3 kcal/mol) gives three compounds upon free radical
chlorination: Compounds B and C are achiral
and (±)-D is not. Exposure of B to aq.
NaOH gives principally product E (C = 72.41%, H =
13.79%) and little F. Compound C reacts
readily with excess water to form G and some
H. On the other hand, treatment of C with
aqueous NaOH affords only H and no G.
Treatment of compound D with the hindered base
t-C4H9OK gives both H and
F. Catalytic hydrogenation of F gives A
with the liberation of 30.0 kcal/mol of heat. The heat of
hydrogenation of H is -25.5 kcal/mol. |
|
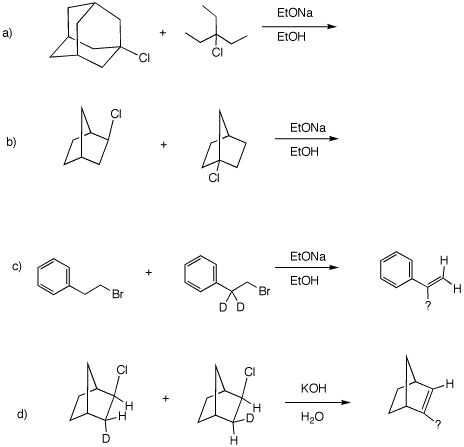
4. In all of the following reactions, a limited amount of reagent is employed. In parts a) and b), which of the starting reactants disappears faster. Explain. In c) and d), what is the structure of the product and how and why is it formed. Use curved arrow mechanisms where applicable.
|
|
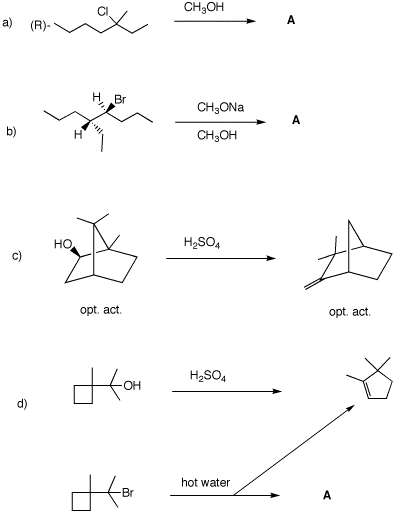
5. In each of the following questions, provide the missing structure with a mechanism for its formation. In cases where a product is provided, show a mechanism for its formation. Pay attention to optical activity and stereochemistry.
|