|
2. Terpenes are naturally occurring
compounds that are comprised of multiples of the
C5 unit isoprene (it looks like 2-methylbutane).
Limonene is a monoterpene that occurs as both enantiomers in
nature. The (R)-enantiomer has an orange, citrus-like
aroma while the (S)-enantiomer has a harsher, lemony
fragrance.
a) Of the limonenes shown on the right,
identify the R and S enantiomers.
b) (R)-Limonene (d-limonene) is reported
to have a rotation of [α]D
123.8o. Its enantiomer is reported as [α]D
101.3o. Assume that the enantiomer with the lower
rotation is contaminated with the other enantiomer,
calculate the percent of (+)- and (-)-enantiomers in the
sample.
c) When compounds containing double bonds
are treated with H2 in the presence of a noble
metal catalyst, hydrogen is added to the double bond. In the
case of (R)- and (S)-limonene, compounds A and
B are formed, both with the formula
C10H20. Are A and B
formed in the same ratio?
|
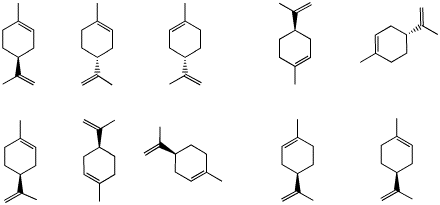
d) The energy difference between the
chair conformations of A is greater than the energy
difference of the chair conformations in B. What are
the structures of A and B? What are the energy
differences? Explain and illustrate the equilibria involved.
Show work. [Use values posted on the Bulletinboard.]
|