Potassium cation solvated by the
cyclic polyether, 18-crown-6 [18-membered ring; 6
oxygen atoms]. Each of the ethano groups is in a
staggered conformation with each of the O-C-C-O dihedral
angles at ~60o [gauche]. For a dynamic
version, click
here. Note that the six oxygen
atoms occupy the same spatial arrangement as do the six
carbon atoms in chair cyclohexane. The discovery of the
crown ethers by Charles
Pedersen of DuPont earned him
a share in the 1987 Nobel Prize in
Chemistry.
|
|
1. Compound (R)-A,
C7H14O2, reacts with
excess Grignard reagent B to afford two products,
C
[(R)-C4H10O] and
D (C7H16O). Compound
D is inert to Jones reagent, but it readily reacts
with cat. H2SO4 to form only
E,
C7H14.
Treatment of compound E with cat.
OsO4/HIO4 produces F and
G. Compound F gives H,
C2H4O2, upon exposure to
Jones reagent, while compound G is inert toward
this reagent. What are the structures of A-H?
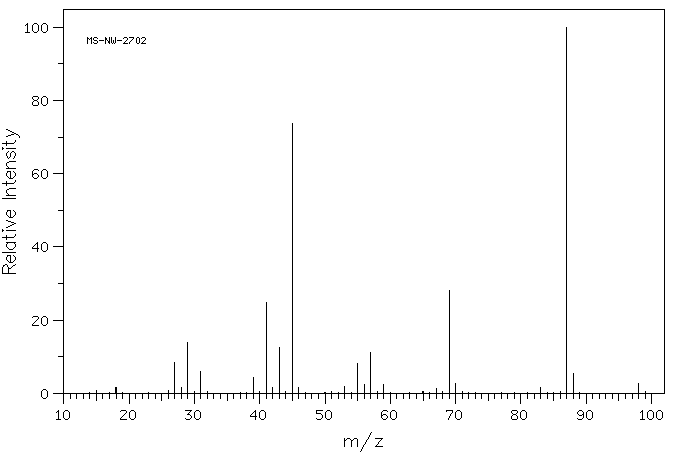
Explain m/z = 45 and 59 in the mass spectrum of C.
Why is m/z = 45 more intense than 59? Explain the base
peak in the spectrum of D.
|
|
Compound D
|
Compound C

|
|
2. Provide reagents for the chemical
transformations shown on the right. Some conversions may
require more than one step.
3. Show how the Grignard reagent RMgX can be parlayed in
one step into the following alcohols: RCH2OH,
RCH2CH2OH,
RCH2CH2CH2OH, and
RCH2C(CH3)2OH.
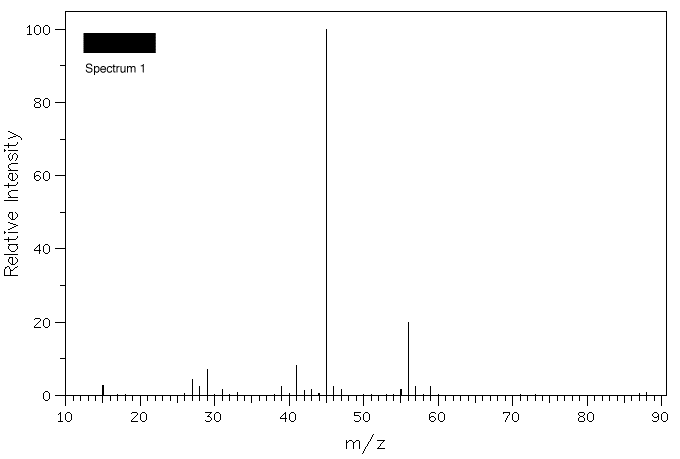
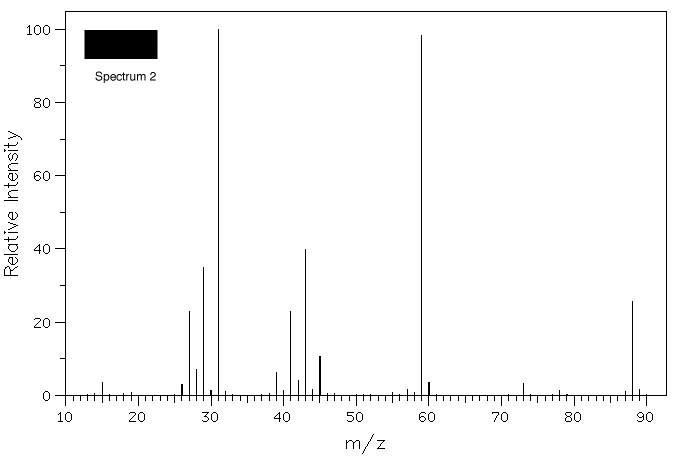
4. Three samples of ethers, ethyl-n-propyl ether, n-butyl
methyl ether, and sec-butyl methyl ether, have lost their
labels. A student records the mass spectra of the
compounds and assigns their structures. Assign the
spectra below to its ether based upon your knowledge of β-fragmentation.
Click on the spectrum for a blow-up.
|

|
|
5. Explain the molecular
ions for trichloroethylene shown in the mass spectrum
below. How do the intensities compare with theory? Click
on the spectrum for a blow-up.

|