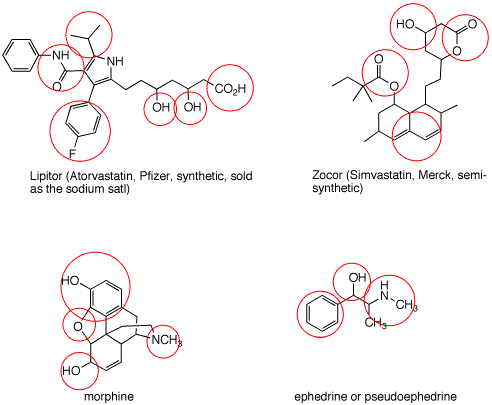
|
1. In 1818,
William
Prout, MD, presented a
paper
before the Royal Society of London on the oxidation of uric
acid (1), the cause of gout. Treatment of uric acid
with nitric acid formed purpuric acid 2. Treatment of
purpuric acid with ammonia gave a purple ammoniumsalt
3, which was used as a dye (Murexide).
Prout conducted a combustion analysis on 3 and found
the percentages of carbon, hydrogen, nitrogen and oxygen
found on page
423.
a) Why do these four percentages add up
so closely to 100% (99.98%)?
b) What atomic masses did Prout use to
determine the number of each type of atom?
c) In determining the ratio 2:2:2:1, is
there anything suspicious about the numbers?
d) Knowing what you know from general
chemistry, what would you suggest as the simplest empirical
formula for 3, based upon the data
presented?
e) Of course, Prout had no idea about the
structure of 3. However, compare his empirical
formula with the molecular formula derived from 3.
How well did he do?
f) What is the structure of purpuric
acid?
g) Purpuric acid has a pKa=0.
What does this value mean?
h) Using resonance structures, show why
you would expect purpuric acid to be more acidic than
acetic
acid.
|