(but don't wait 'til the last minute)
|
The topic of oxidation levels of
organic compounds is addressed in passing on pg. 446. We
will consider the issue in more detail as described in
Oxidation
Levels.The alcohol module in
ORGO
will give you a good review of some of the fundamental
reactions discussed in class and in Chapters 10 and
11. 1. (20 pts) Compound A (C6H12O2) reacts with 2 equiv. of an aliphatic Grignard reagent B to produce a single compound C. Treatment of C with H2SO4 readily affords only D, which upon ozonolysis gives E and formaldehyde. What are the structures of A-E? Explain and illustrate. |
Victor Grignard (1871-1935) |
|
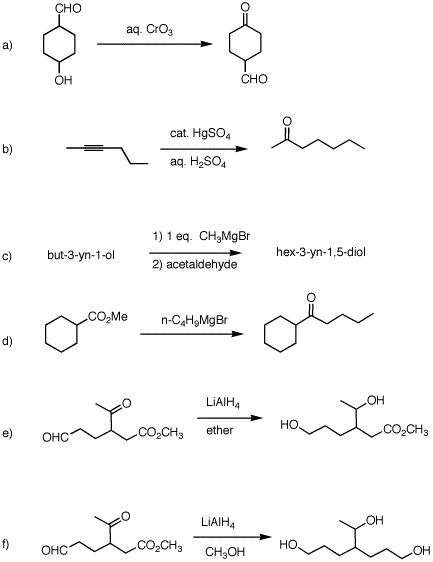
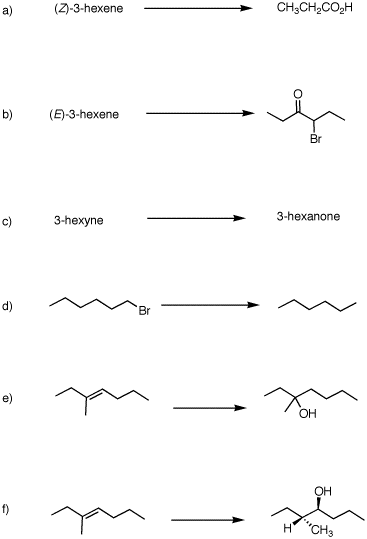
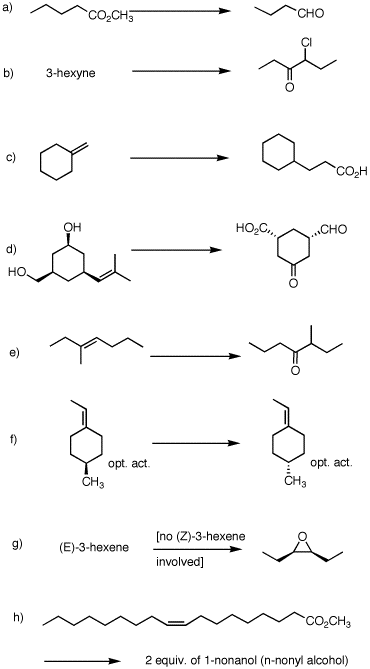
2. (40 pts) Provide reagents and intermediate compounds in the following transformations. Each reaction requires more than one set of conditions.
|
|
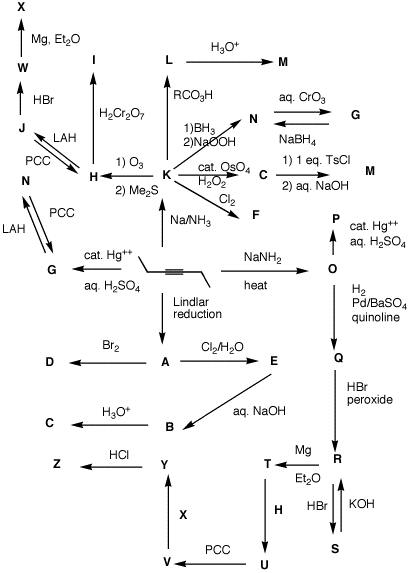
3. (100 pts) The Maze of Reactions: Provide the structures A-Z. Pay attention to stereochemistry and conditions. For additional exercises of this type, see the Web of Reactions. For a single page version of the Maze, click here. |
|
4. (30 pts) How many grams of potassium dichromate are required to oxidize 0.1 moles of ethanol to acetic acid? Show the balanced redox reaction and the rest of your calculations. |
|
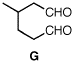
5. (30 pts) An optically-active component ((R)-A) of citronella oil has the formula C10H20O. Treatment of A with PBr3 provides B, which forms Grignard reagent C. Addition of isobutyraldehyde [(CH3)2CHCHO] to C leads to compound D. Treatment of D with H2SO4 gives optically inactive E (C14H26). Oxidation of A with PCC provides F, which upon ozonolysis gives optically active dialdehyde G. What are the structures of A-G? Explain and illustrate. Pay attention to absolute (R vs. S) stereochemistry. |
 |
|
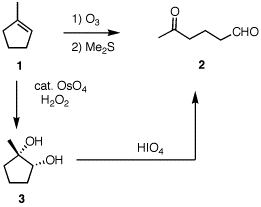
6. (20 pts) Your text (pg. 473) illustrates the sequence of reactions 1 --> 3 --> 2. [The text doesn't specify catalytic OsO4, but it is.] This procedure, which you will notice is the equivalent of ozonolysis (1 --> 2), is a two step procedure; the diol 3 is isolated. Explain what will happen if HIO4 replaces H2O2 as the stoichiometric reagent. What about the use of KMnO4 in place of H2O2? Assume that HIO4 and KMnO4 can oxidize Os(VI) to Os(VIII). |
 |
7. (60 pts) Each of the following
reactions has a fatal flaw. What are they? How would you
change the conditions of each reaction to accomplish the
transformations? 8. (60 pts) In each of the following
reactions, determine whether the process is an oxidation,
a reduction, or an electroneutral process. Give the total
electron change in each case and provide the reaction
conditions.