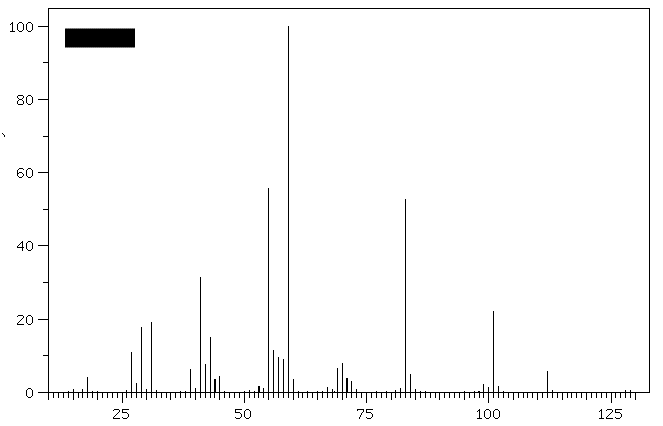
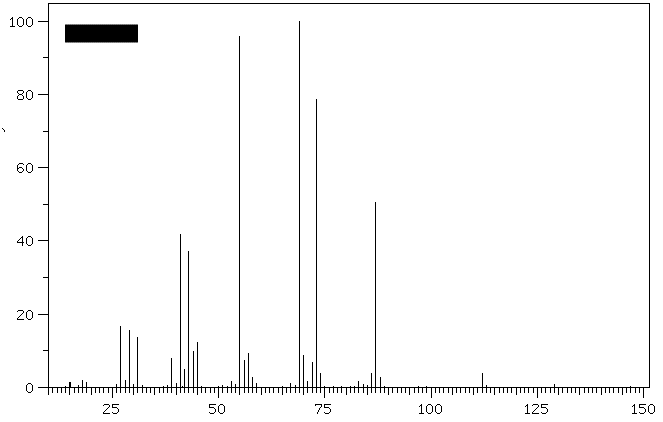
1. (30 pts) Treatment of compound
A with catalytic HgSO4/aq.
H2SO4 gives two constitutional
isomers B and C. Exposure of B and
C independently to NaBH4 affords
compounds D and E, respectively.
Hydrogenation of A provides n-octane. a) What are the structures
A-E? b)What is the molecular ion for
D and E and is it visible in the
spectra? [For larger versions of the
spectra, click
here.] 2. (10 pts) (S)-Epichlorohydrin is
treated successively with methyl magnesium bromide and
isopropyl magnesium bromide to form chiral compound
A. What is the structure of A and its
absolute configuration? Explain and illustrate with a
mechanism. 3. Thedata to the right represent the
fragmentation patterns of two acyclic alkanes. What are
their structures? Explain and illustrate? How many
compounds are eligible for consideration? Which one is
eliminated? Estimate the intensity of M+1=73. c m/z intensity 15 2 26 3 27 43 28 5 29 60 30 1 37 1 38 3 39 30 40 5 41 88 42 95 43 100 44 6 50 2 51 2 53 3 55 10 56 40 57 94 58 5 71 4 72 16 c m/z intensity 26 1 27 22 28 3 29 20 38 1 39 12 40 2 41 50 42 78 43 100 44 4 53 1 55 4 56 3 57 20 72 17 4. (40 pts) Provide the missing
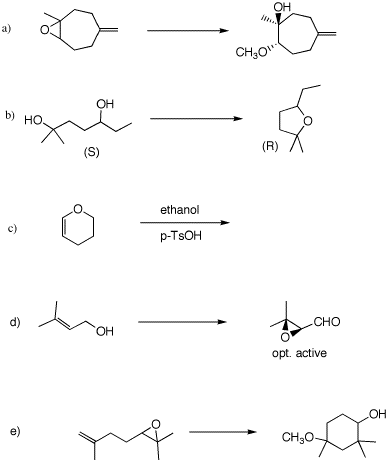
information in each of the following reactions. Provide
explanations and mechanisms. For 4d, see pg.
621. 5. (50 pts) Provide the structures of
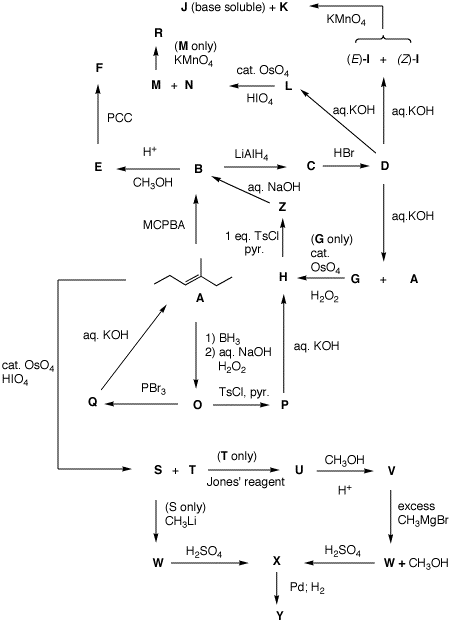
A-Z. Show your reasoning where it is called for.
Be brief.