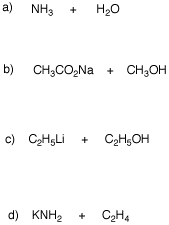|
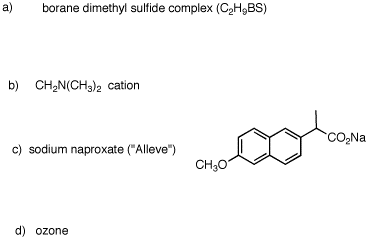
1. For each of the following substances, identify the functional groups present in each one. [Functional groups are listed on the inside front cover of your text.] Draw line-angle formulas for b-e and g. 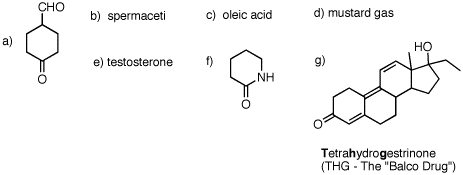 |
|
2. Ever since Lavoisier established the importance of gravimetric analysis and the principle of the conservation of mass in chemical reactions, chemists have been able to determine the mass of carbon and hydrogen in organic compounds. While combustion gives carbon as CO2 and hydrogen as H2O, oxygen is determined by difference. The problem was that there was no universal acceptance through the late 1800's as to the mass of carbon (6 or 12?) and oxygen (8 or 16?). In 1852, Alexander Williamson reported on the formation of a mixed ether, ethyl methyl ether. Fortunately, Williamson subscribed to the scheme H=1, C=12, and O=16. [Reading Assignment: A Brief History of Organic Chemistry.] Using his data on pages 232-233 in the accompanying paper, confirm the empirical formula obtained by Williamson given the mass of the sample and the mass of combustion products. Using the vapor density of the ether compared with the density of air, whose effective "molecular weight' is known, confirm the molecular formula of the ether. You need not use the data relating to the "globe". |
|
3. For the hydrocarbon shown below, identify the hybridization of each of the carbon atoms. Redraw the structure showing p-bonds with p-orbitals. 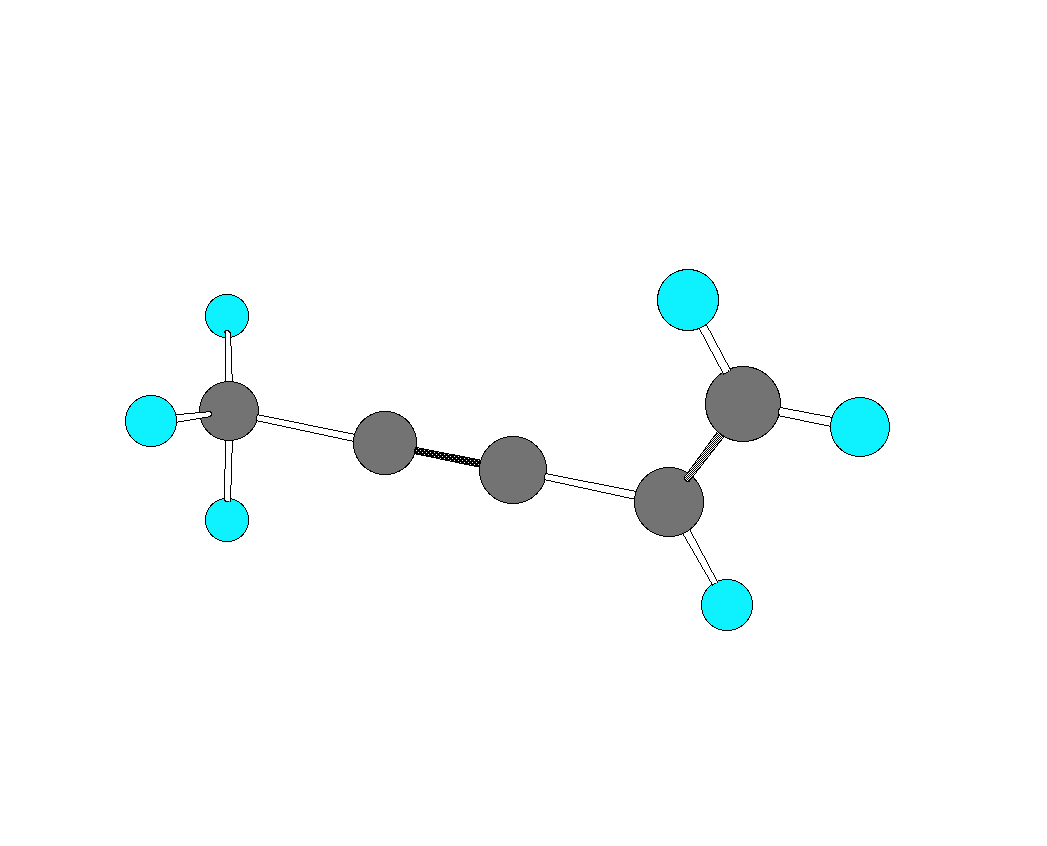 |
|
|
4. For each of the following acid-base reactions, provide the products of the reaction (conjugate acid and conjugate). Provide equilibrium arrows to show the direction of the equilibrium. Use the data in the pKa table to determine the direction and magnitude of the equilibria.
|
|
5. For each of the species listed below, draw Lewis structures. Draw bonds as lines and non-bonded electrons as a pair of dots. Provide charges and resonance structures where applicable.
|
|
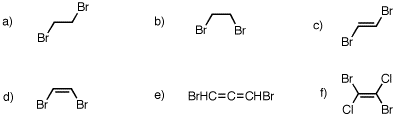
6. Which of the following compounds have a net dipole moment? Explain and illustrate.
|