Chem 220a Problem
Set 6 Fall 2003
1. 0.01 M CH3CH2CH2CH2Br reacts with 0.01 M NaCN in ethanol to yield primarily CH3CH2CH2CH2CN, while 0.01 M (CH3)3CBr reacts under the same conditions to give primarily (CH3) 3COCH2CH3. Explain concisely.
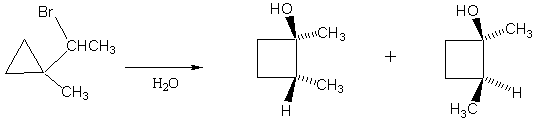
2. Explain the following observation. Why is there only the one product?
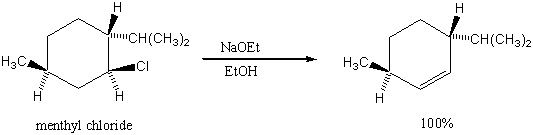
3. Why is the Saytzeff product not formed in the following reaction?
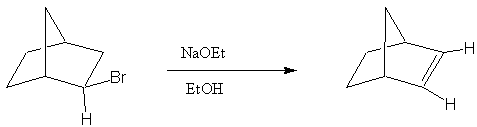
4. Provide a reasonable mechanism to explain the following observation.