Chem 220a Problem
Set 5 Fall 2003
1. Arrange the compounds of each set in order of reactivity toward (i) SN2 displacement and (ii) E2 elimination by KOH in ethanol.
(a) 2-bromo-2-methylbutane, 1-bromopentane, 2-bromopentane
(b) 1-bromo-3-methylbutane, 2-bromo-2-methylbutane, 2-bromo-3-methylbutane
(c) 1-bromobutane, 1-bromo-2,2-dimethylpropane, 1-bromo-2-methylbutane
2. Predict the organic products of the following reactions. Label as major and minor, if multiple products are likely.
(a) Isopentyl bromide + NaOEt in EtOH
(b) Isobutyl bromide + NaOEt in EtOH
(c) Methyl bromide + KO-t-Bu in t-BuOH
(d) t-butyl bromide + KOCH3 in CH3OH
(e) 2-bromo-3-methylbutane + NaOEt in EtOH
(f) 2-bromo-3-methylbutane + KO-t-Bu in t-BuOH
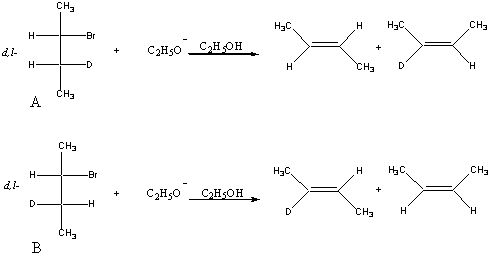
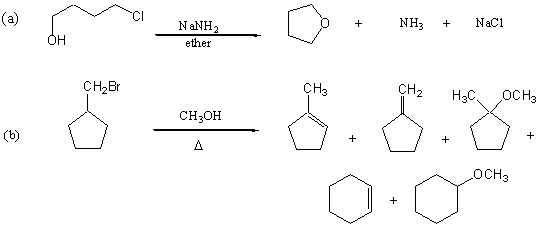
3. Provide reasonable mechanisms to account for the following observations.
4. Starting with isopentane, devise efficient syntheses of (a) 2-methyl-2-butene and (b) 2-methyl-1-butene. Any common reagents may be used.
5. Explain the following observations.