Chem 220a Problem
Set 2 Due
- Given the boiling pint of the first compound in each pair, estimate the boiling point of the second compound.
(a) CH3-CH2-CH2-CH=CH2 (bp 30°), CH3-CH2-CH2-CH2-CH=CH2
(b) CH3-CH2-CH2-CH2-CH2-CH2-Br (bp 155°), CH3-CH2-CH2-CH2-Br
- Draw the structures and give the IUPAC names of all the isomeric heptanes. There are 9.
- Draw the structures and give the name of an alkane that
(a) has more than three carbons and has only primary hydrogens.
(b) has seven carbons and has only secondary hydrogens.
(c) has a molecular weight of 84.2.
- An elemental analysis of an amide with molecular weight 87 shows it contains by weight 55.14% carbon, 10.41% hydrogen, and 16.08% nitrogen. What are the possible structures for the compound?
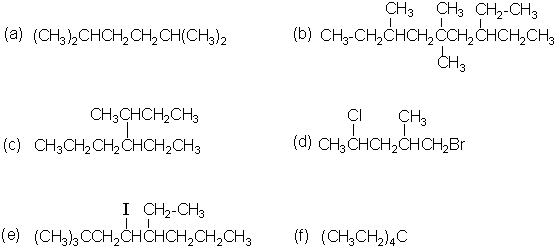
- Give the IUPAC name for each of the following compounds.
- (a) Draw Newman projections for the six staggered and eclipsed forms of 2,3-dimethylbutane obtained by rotation about the central (C2-C3) bond.
(b) Estimate the relative energies
of these conformers and sketch the graph of dihedral angle vs. relative energy.
(The relative energies should be estimated to one decimal place, e.g., 3.2 kcal/mol.)
7. Draw the most stable chair conformation for each of the four isomeric 1,3,4-trimethylcyclohexanes.