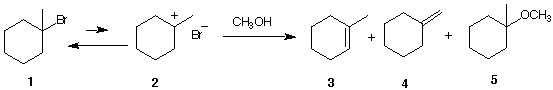Chem 220a
Problem Set 5
Chapter 6
Due: Monday, October14,
2002
|
1. Study #2 and #3 in the Alkyl Halide
module and #1 in the Ether module in ORGO.
2. When
2-bromo-1,1,1-trideuteriopropane is heated with
C2H5ONa in ethanol, the major olefin
formed is CH2=CHCD3. Why? What is the
structure of the minor olefin?
3. Compound A, C7H16, forms
three monochloro constitutional isomers (B, C,
and D) upon free radical chlorination. Compound
B readily gives E, C7H16O,
upon treatment with aqueous NaOH. Compound C forms
two compounds F and G (both
C7H14) under the same reaction
conditions. Compound D reacts readily with water to
give H (C7H16O) while its
reaction with aqueous sodium hydroxide affords a single
compound G. What are the structures
A-H? Explain. [Hint: How many of the nine
heptanes (can you draw them?) form 3 monochloro compounds?
The rest of the information reduces the possibilities for
A to a single compound.]
|

Biot examining Pasteur's tartrate
crystals
|
|
4. (3R,4R)-4-Bromo-3-methylheptane
(A) reacts with
C2H5ONa/C2H5OH
to form an optically inactive compound B. Either
enantiomer (C and D) of the diastereomer of
A, forms E under the same reaction
conditions. Compound E is also optically
inactive. Compound C reacts with
C2H5SNa to form a sulfide F of
the 3R, 4R configuration. Explain and illustrate.
|
|
5. A graduate student makes the
predictions shown on the right about optically active alkyl
bromides 1 and 3 with strong base. Use your
knowledge of the conformation of cyclohexanes and E2
elimination reactions to answer the following questions.
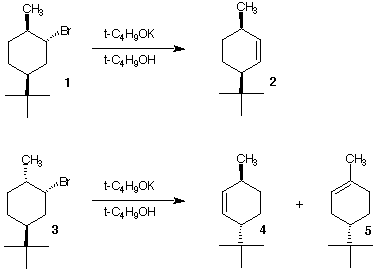
a) Would the same prediction apply if
1 and 3 were racemates?
b) Are the alkenes optically active?
c) Why is only alkene 2 predicted in the reaction of
bromide 1 while bromide 3 is predicted to
afford alkenes 4 and 5?
d) Is alkene 4 or 5 expected as the major
product?
e) What is the relationship between bromides 1 and
3? Alkenes 2 and 4? Alkenes 4
and 5?
|
|
6. The solved problem on pg. 279 is
reproduced below. Ether 5 is the major prodct of the
reaction while alkenes (olefins) 3 and 4 are the minor
products of the reaction. Of these two olefins, compound 3
is the major one.
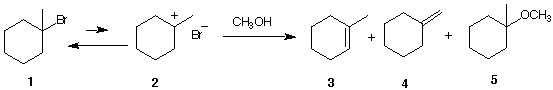
a) What is the other product of the
reaction?
b) What are the mechanisms by which 3, 4, and
5 are formed?
c) What if you were told that the ratio 3/4 is not
necessarily the same early in the reaction (say 10% of
1 consumed) as it is after the reaction is complete.
Respond. [Hint: Look at the arrow under methanol and
consider your answer to 5a.]
d) If stoichiometric sodium methoxide were used along with
methanol in this experiment, the ratio 3/4 is
predicted to be the same during the course of the reaction
and ether 5 is not among the products of the
reaction. comment.
e) In 5d, is 3 or 4 expected to be the major
product? Explain.
f) What mechanism is operative in 5d?
g) What would you expect to happen to the ratio of 5d if the
basic conditions of problem 4 were employed instead of
CH3ONa/CH3OH?
h) Show how ether 5 can be formed by an
SN2 mechanism.
|
|
7. Provide reactants or products for the
following reactions. Comment and illustrate.

|