Chem 220a - Organic Chemistry
Problem Set 2
Chapter 3
Due: Monday, September 23, 2002

|
Adolf
von Baeyer (1835-1917)
Baeyer's ideas on Strain
Theory predicted that
cyclopentane was less strained than cyclohexane. Because
there was only one cyclohexane carboxylic acid, he concluded
that there could not be both an equatorial and axial
isomer.
Equatorial is often misspelled. Witness the map from the New
York Times (5/23/00)
|
|
How
to Draw Cyclohexanes (PowerPoint)
1. The Conformation
Module in the Study Aids will give you a
good overview of the subject of conformation. Work your way through
it. (You will need ChimeTM
to view the Module). [How
to manipulate Chime
structures].
2. Redraw (line angle formula) and name (IUPAC)
the hydrocarbon in this problem. For a dynamic view click
here.
For a static view click here.
Get ChimeTM
here.
3. The DGo
= 0 kcal/mol for the difference in energy between the two identical
chair conformations of trans-1,3-dimethylcyclohexane. In what way, if
any, do they differ? On the other hand, the energy difference between
the two chair conformations of cis-1,2-dimethylcyclohexane is also
DGo
= 0 kcal/mol but they are isoenergetic (same energy) and not
identical. In what way do they differ? [Note: Did you do #1?]
4. The four 1,2,4-trimethylcyclohexanes shown
below are identical with one another.
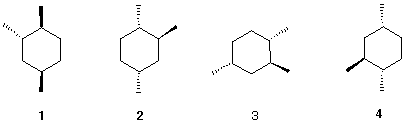
a) Assemble a molecular model and
convince yourself.
b) Imagine structure 1 at the origin of
an xyz-axis system (x = horizontal, y = vertical, z =
perpendicular to xy plane). What rotations are required to convert
1 into 2? 1 into 3 ? 1 into
4?
c) Determine DGo
for the difference in energy between the chair conformations of
this 1,2,4-trimethylcyclohexane. Illustrate and explain.
5. Two 1,4-disubstituted cyclohexanes A and
B are stereoisomeric. Compound A has an energy
difference of 2.3 kcal/mol between its two chair conformations while
compound B has a difference of 1.3 kcal/mol between its two
chair conformations. What are the structures of A and
B? Explain. [See pg. 118]
6. Determine the percentage of chair equatorial and axial
isopropylcyclohexane present at 25 oC.
[DGo
= -RTlnKeq; R = 1.98 cal/mol-deg K. There are three
staggered rotamers of the isopropyl group within the equatorial
isomer. Assume that CH3/CH2 staggered = 0.9
kcal/mol , what is the energy difference between the two (two of them
are isoenergetic)? Show calculations and illustrate.

